Patient Has At Least 20 Cysts Removed From Her Neck! | Dr Pimple Popper: This Is Zit 1110
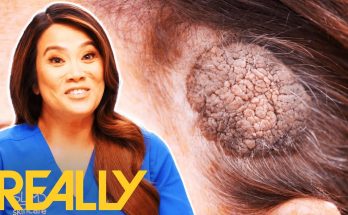
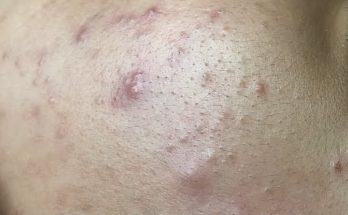
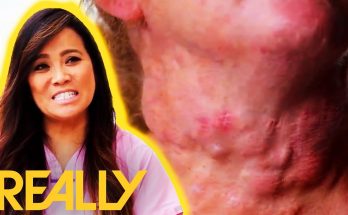
Dr Lee performs a difficult surgery on patient Audrey that requires more than one trip in order to remove at least 20 cysts from Audrey’s neck.
Patient Has At Least 20 Cysts Removed From Her Neck! | Dr Pimple Popper: This Is Zit 1110 Read More